Laboratory
Antufen Seed’s laboratory is located in Pichidegua, in the province of Cachapoal, 100 miles south from Santiago. This facility is intended to carry out germination analyses, weed detection and identification procedures and evaluations of eventual and assumed pathologies. Aforementioned studies and processes are applied with the purpose of ensuring a final product which fulfills the genetic quality and phytosanitary requirements demanded by our customers. With the intentionto prove the high quality of our products through germination and physical purity tests and phytopathological analyses, the laboratory facilities are fully equipped to perform said processes. Address: Parcela 11-12 Santa Elisa y San Pedro S/N, Pichidegua – Cachapoal.
-
Pathology
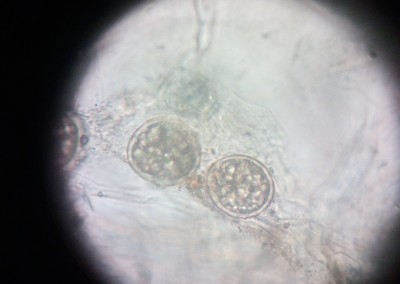
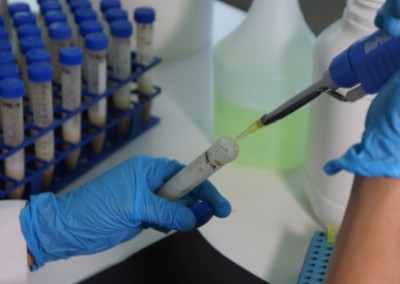
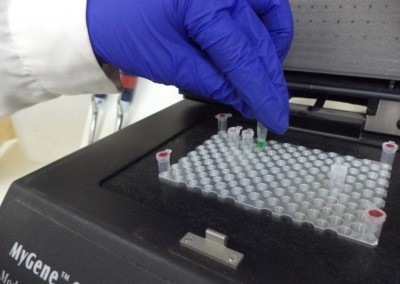
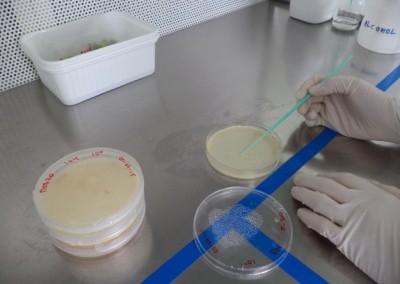
The Pathology Team is continuously present in the different stages of the production process, doing its best effort to achieve the expected final product. In line with this objective, different analyses to parent seeds are performed, regular sampling to nurseries are taken and sample gatheringsof our hybrid seeds to verify the absence of diseases are regularly carried out. Our highly specialized staff and the cutting-edge laboratory equipment we have available, permit us the detection of viruses, bacteria and fungi and particularly seed borne diseases. In the laboratory premises, differential and semi-selective culture media to detect fungi and bacteria are developed; serological tests (Inmunostrips and ELISA) to determine the existence of viruses and some bacteriaare implemented andmolecular analyses for some bacteria and viruses are carried out. All this complemented with pathogenicity and hypersensitivity tests performed in differential hosts. Every and each seed analysis is carried out in accordance with ISTA protocols
-
Germination and Physical Purity
Germination and Physical Purity Tests to seeds are conducted to every seed lot. These seeds quality characteristics tests are carried out following the ISTA protocols and in compliance with AOSA regulations and methods.